Organ of the month: Skin
I. Indications for skin biopsy
Skin biopsies account for a significant percentage of a typical diagnostic laboratory’s caseload. The main reasons for this is that skin disease is common and, as an organ system, the integument is easily visible to the owner and clinician. Biopsy is also relatively straightforward in comparison to most other body systems. There are many indications for biopsy since any skin lesion or mass should be biopsied, particularly if failing to resolve with treatment, or progressing. Ulcerative and vesicular diseases should be biopsied. Biopsy is also likely to be one part of a broader diagnostic approach including hair plucks, tape testing, bacterial and fungal cultures, and cytology.
II. How to sample and send skin samples 1
The two main types of biopsy relevant to the skin are excisional and incisional. Excisional biopsies are more likely to be used to excise appreciable masses, whereas incisional biopsies are more commonly used to investigate dermatopathies. This category includes punch biopsies. For punch biopsies, there are a few golden rules to bear in mind:
a) It is fine to clip long hair with scissors but please do NOT rub, scrub or vigorously clean the skin surface – this risks losing potentially diagnostic surface material.
b) For cases with more than one lesion, take four samples using a 6mm punch. Use a fresh punch for each new case, avoid dull punches that traumatise the sample, and use the tool to cut rather than placing added pressure. [Note: We charge the same for up to five skin punch biopsies]
c) For focal lesions, two punch biopsies should be sufficient.
d) For nasal planum and footpad lesions, 4mm punches are best.
e) Avoid compression and crush artefact by scooping the sample rather than picking it up with anything such as forceps.
f) Place directly in 10% neutral buffered formalin. Do this immediately to avoid drying out. Cassettes should be avoided as they cause compression artefact too (they will turn your carefully collected sample into a waffle).
g) It is best to sample lesions earlier in the disease course (or at least a range of representative lesions). Sampling more longstanding cases carries a higher risk of capturing chronic features common to lots of conditions but specific to none. There is no need to sample normal skin and try to avoid areas of self-trauma.
h) Consider submitting one punch fresh for culture.
i) We do occasionally receive punch biopsies from known mast cell tumours, e.g. following FNA. A punch biopsy of a mast cell tumour does not really progress exploration of the case as it does not allow margin assessment or, in many cases, accurate grading. The best approach, where feasible, is complete excision and submission of the entire tumour for histopathology.
Excisional biopsy
a) For ulcers and bullae, an ellipse is better than a punch biopsy. For lesions in the subcutis, an excisional biopsy is best. Unless particularly large or likely to require surgical planning, cutaneous or subcutaneous masses should be submitted as excisional biopsies.
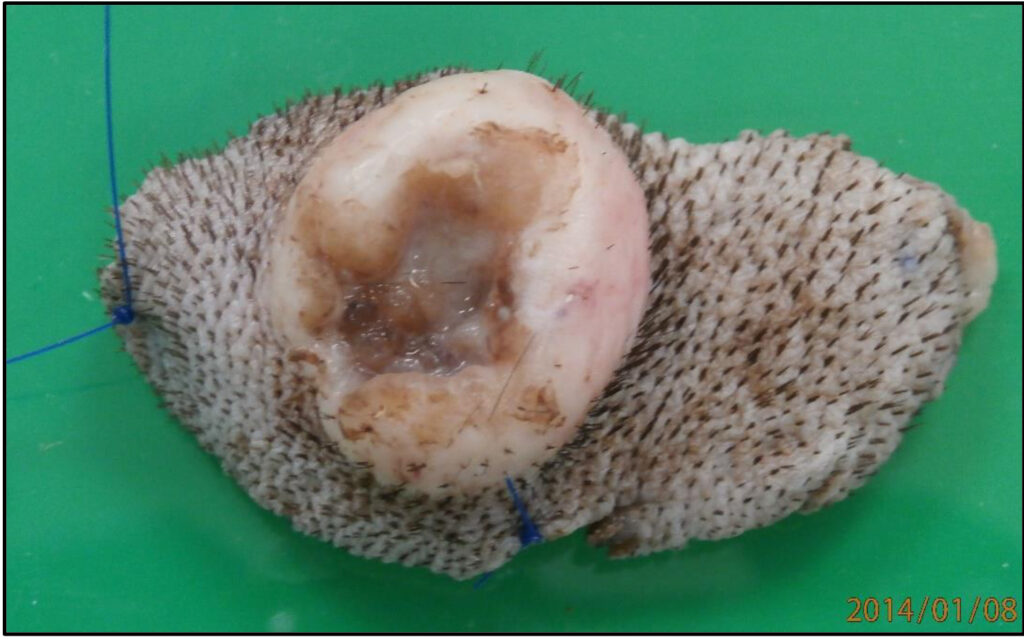
b) If you are interested in particular margins, please label them in some way and let us know on the submission form. You can use different suture lengths for example, or surgical ink if you have some (not normal ink, which does not survive processing). Adding a diagram to the submission form or sending images can also be very helpful. See Fig 1 below.
c) It is best to avoid making an incision into the mass. However, if you feel you need to do this to allow formalin penetration, please make the cut from the skin surface, not the deep border, as this can hamper evaluation of the deep margin. Fact sheets you may find helpful are available on our web site (please see References). They include how to send particularly large samples.
III. Relevant clinical information
Signalment and clinical history should always be included with skin (any!) biopsies. A brief, pertinent clinical history is greatly appreciated, rather than the patient’s whole history (the latter risks important information being missed inadvertently). Particularly helpful features are outlined below:
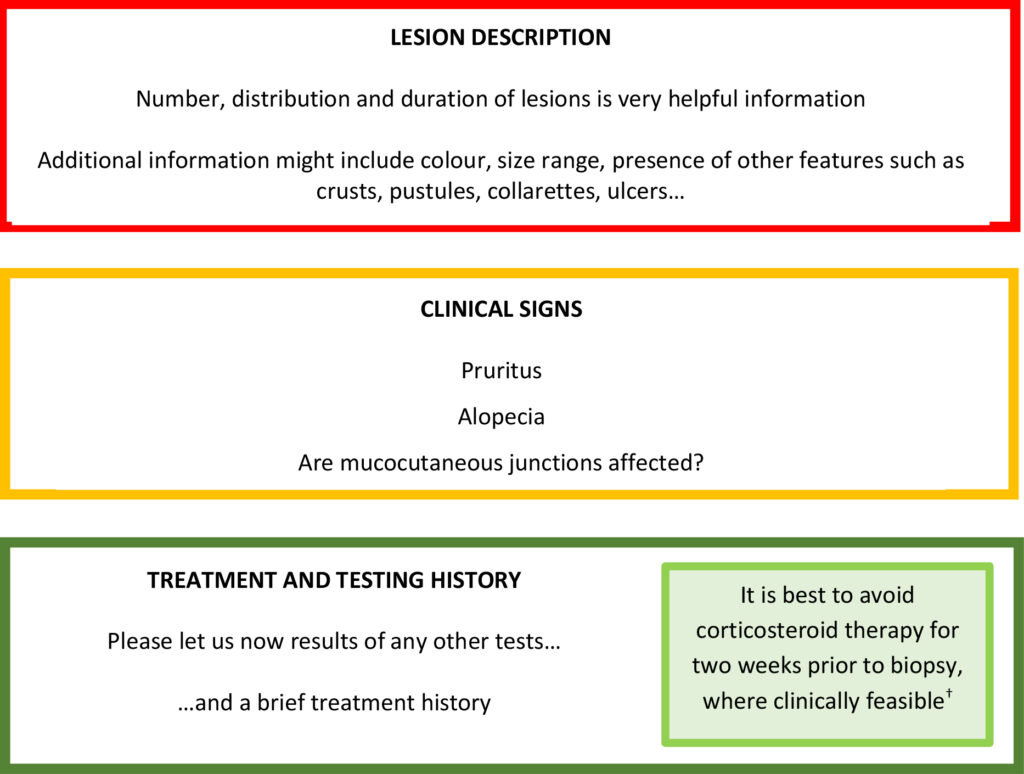
† Please note there is no actual data on steroid withdrawal time in the published literature because studies have never been performed.
For cutaneous and subcutaneous masses, pertinent clinical information is (a) lesion location; (b) speed of onset and duration; (c) type of biopsy (excisional or incisional); (d) any FNA results.
Fig 1. Skin ellipse and mass tagged with suture. Long suture at one end (left) and short suture on one side (bottom).
IV. A few last points
Please bear in mind that a definitive diagnosis is not reached in around one third of cases.
Biopsies may still help to exclude some conditions but disorders such as vasculitis and autoimmune-mediated disease (and even neoplasia) cannot be entirely excluded because samples can still be unrepresentative. All that can be concluded in such instances is that there is no evidence of a particular condition in the samples being assessed.
V. Case examples
Fig 2. Almost normal canine skin – epidermis is mildly hyperplastic.
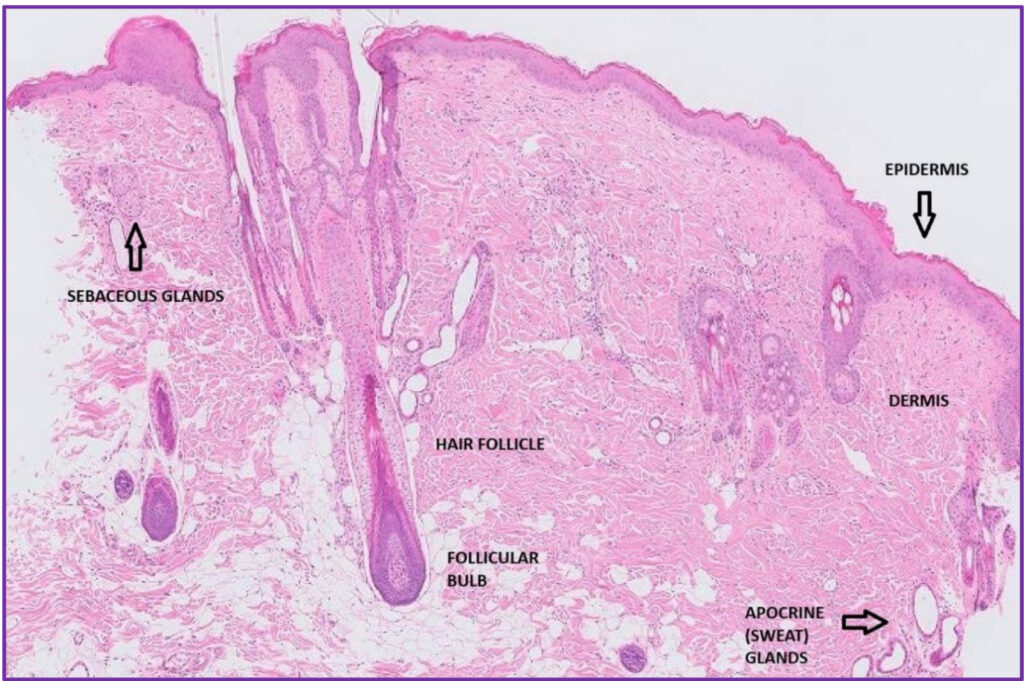
Fig 3. Canine viral papilloma.
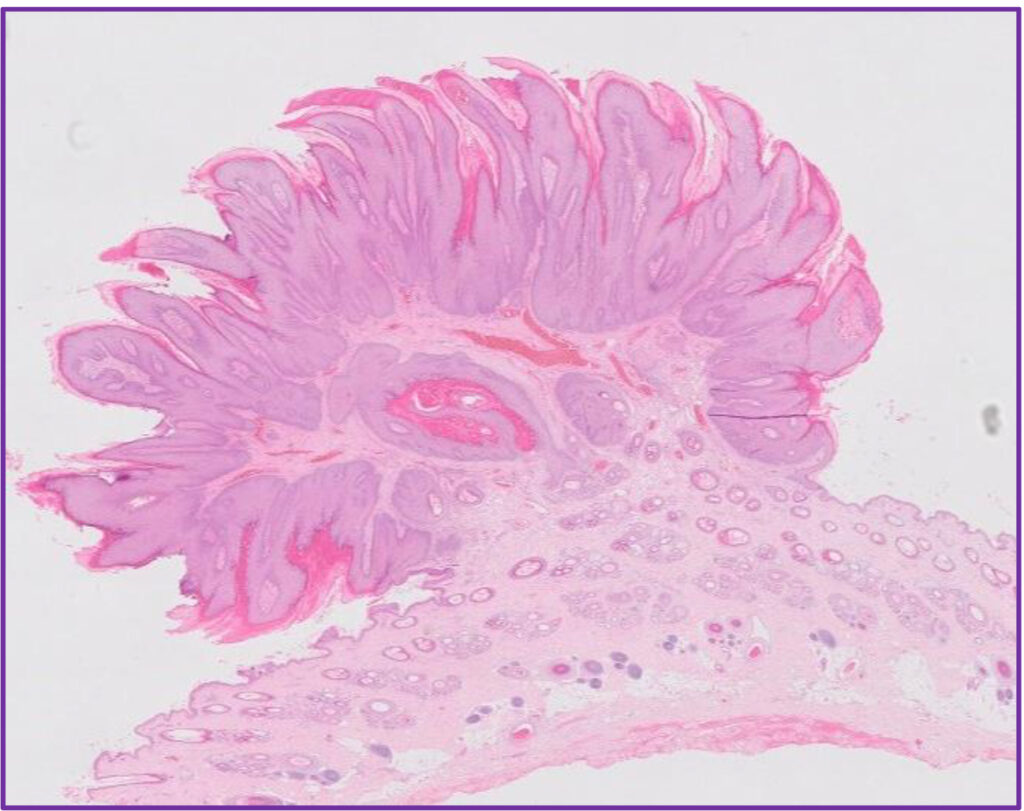
Fig 4. Canine cutaneous histiocytoma.
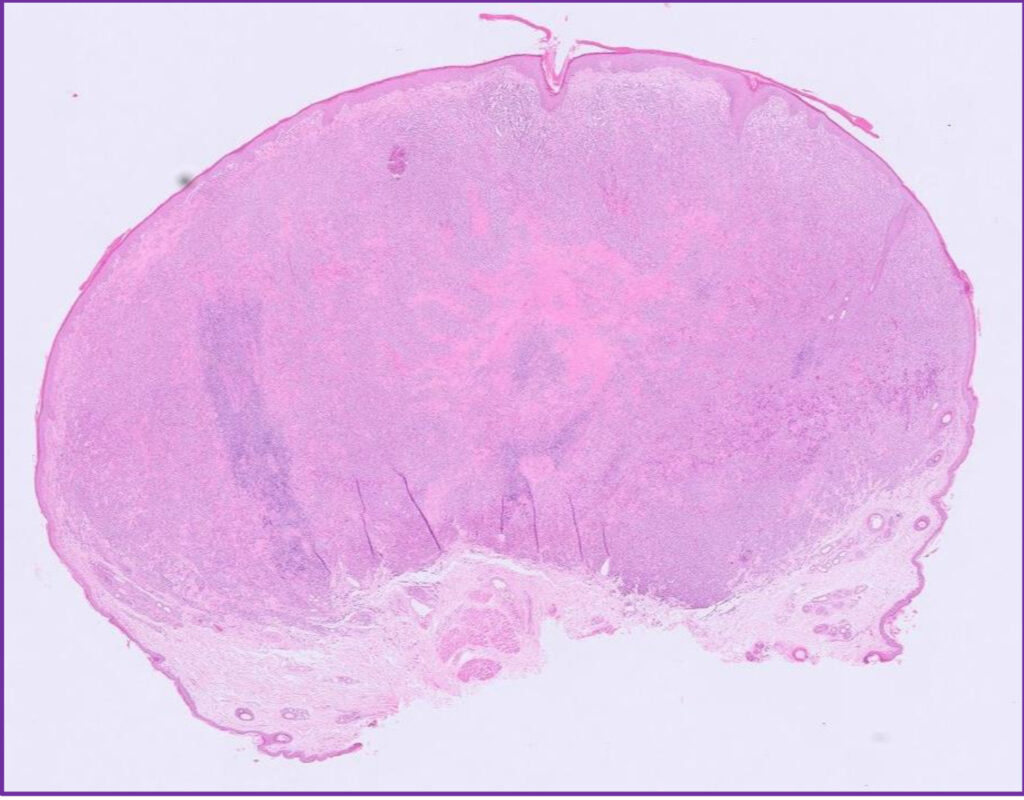
Fig 5. Feline skin. This cat was unlucky as it had two separate skin diseases. Yellow arrows (see Fig 6). Red box – this is hypercellular dermis due to pyogranulomatous inflammation (see Figs 7 and 8).
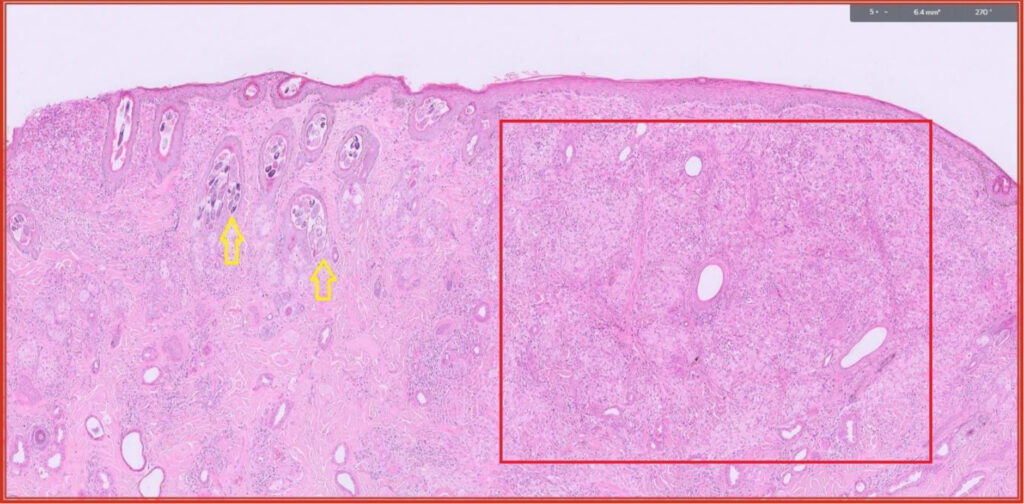
Fig 6. Myriad Demodex mites in multiple hair follicles.
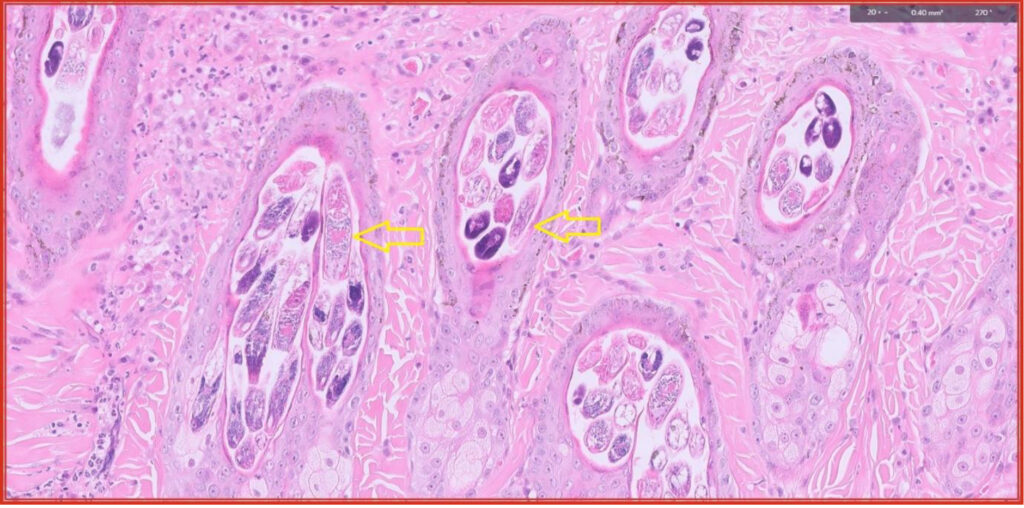
Fig 7. Pyogranulomatous dermatitis with yellow arrows highlighting intralesional fungal organisms (same area as red box in Fig 5).
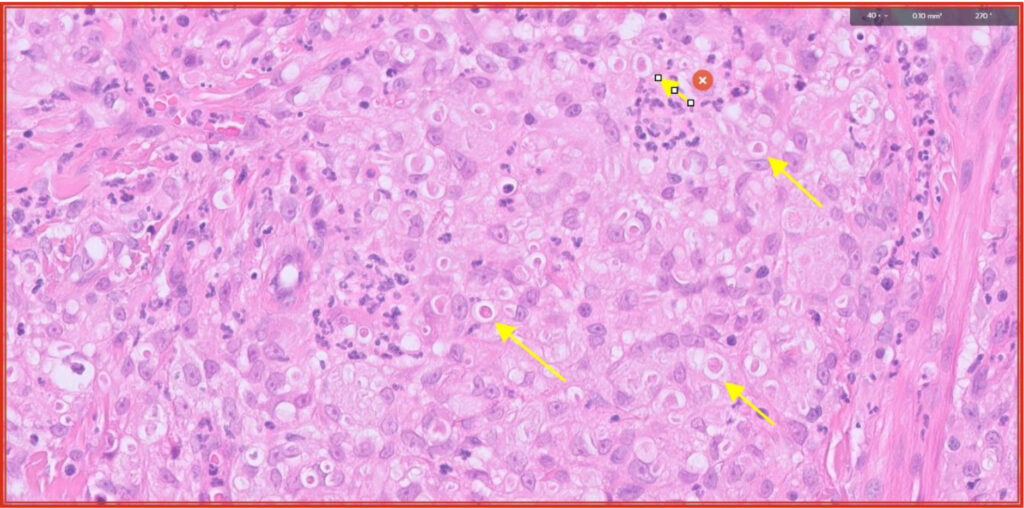
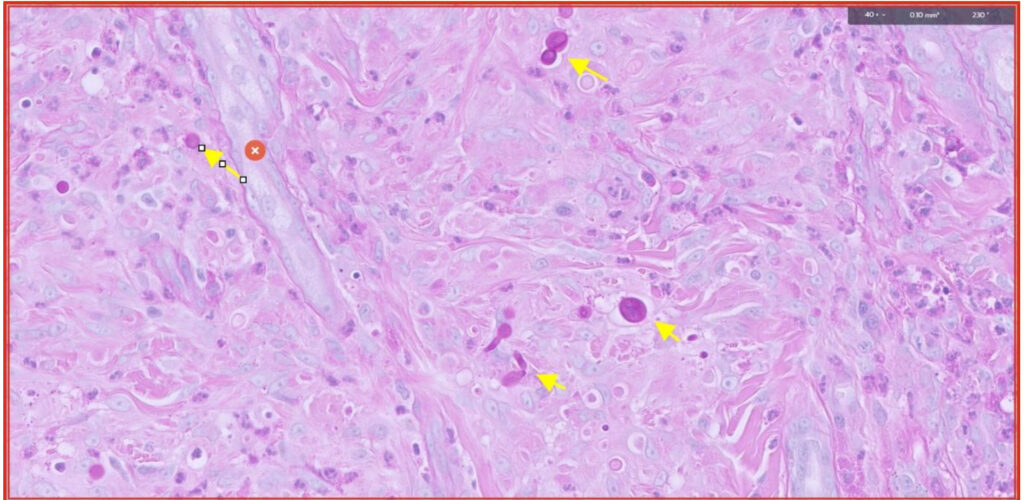
Fig 8. PAS stain highlights the fungal organisms (yellow arrows). They are most likely opportunistic fungi but fungal culture would be required to identify them definitively.
References
- Mauldin E. https://www.vet.upenn.edu/docs/default-source/penn-vet-diagnostic-laboratories/pvdl-before-2019/biopsy-service—optimal-skin-biopsy-technique.pdf?sfvrsn=d22415ba_4
- https://www.finnpathologists.com/wp-content/uploads/2024/11/FINN-Fact-Sheet-1-MCT-Supplemental-Testing-Issue-4.-13.11.2024.pdf
- https://www.finnpathologists.com/wp-content/uploads/2023/11/FINN-Fact-Sheet-9-Submission-of-Challenging-Samples-Issue-2-04.07.2023-1.pdf
- https://www.finnpathologists.com/wp-content/uploads/2023/11/FINN-FACT-SHEET-10-Inking-Issue-3-14.11.2023.pdf
Related Posts
Organ of the Month: Mammary Glands
Organ of the month: Mammary Glands I. Indications for biopsy/pathological evaluation Mammary lesions are among…
Organ of the Month: PART II – The Male Reproductive Tract
Organ of the month: PART II – The Male Reproductive Tract I. Indications for biopsy/pathological…
Diagnosis of Canine Hypercortisolism
Diagnosis of Canine Hypercortisolism Introduction Hypercortisolism is a common endocrinopathy in dogs and is also…
Canine and Feline Blind Bronchoalveolar Lavage (BAL)
Canine and Feline Blind Bronchoalveolar Lavage (BAL) Common indications for blind BAL: Chronic cough Chronic…